See separate guidance for synergistic use in bacterial endocarditis and bone infections. This guideline is not for use in surgical prophylaxis; pregnancy; major burns; cystic fibrosis; ascites; end-stage renal disease or in those receiving haemodialysis or haemofiltration.
The guideline is based on simulations of predicted concentrations using individual estimates of creatinine clearance and volume of distribution (derived from a pharmacokinetic analysis of patient demographic and clinical data, doses, times and concentrations).
Contra-indications to gentamicin therapy: hypersensitivity, myasthenia gravis
Take particular care:
o In those with decompensated liver disease; increased risk of renal failure
o In patients with known or suspected acute kidney injury ( 50% increase in baseline serum creatinine or oliguria > 6 hours in previous 48 hours)
o In patients with chronic kidney disease (CKD Stage 4 or more; eGFR less than 30ml/min/1.73m2).
o If creatinine is currently rising; ensure that creatinine result is up to date.
If there is no alternative to gentamicin, give a single dose and check with Microbiology, Infectious Diseases (ID) Consultant, or Specialist Consultant before giving a second dose.
Toxicity with gentamicin is more likely in septic patients or those who are also on other potentially neurotoxic and/or nephrotoxic drugs, regardless of initial creatinine clearance; review therapy and consider withholding nephrotoxic drugs. Avoid co-administration of gentamicin with
o neuromuscular blockers
o other potentially nephrotoxic or ototoxic agents such as NSAIDs, ACE inhibitors
o potent diuretics
o other aminoglycosides
Consult Summary of Product Characteristcs (eSPC) for a full list (www.medicines.org.uk )
Limit duration of treatment with gentamicin to minimise toxicity. Review all prescriptions daily in conjunction with microbiology results; and continue beyond 72 hours only on the advice of a Microbiologist, ID Consultant or Specialist Consultant.
Vestibular and oto-toxicity due to gentamicin is independent of serum gentamicin concentration and can present with any of the following symptoms: dizziness, unsteadiness on feet, tinnitus, bobbing oscillopsia (vertical bouncing of surroundings), hearing loss, nausea or vomiting. Toxicity is due to drug accumulation within the inner ear and is associated with prolonged aminoglycoside use (usually with durations beyond 10 days but toxicity may occur after 72 hours of therapy). Patients receiving therapy beyond 72 hours should be warned of this potential side-effect and gentamicin should be discontinued at the earliest sign of toxicity.
Patients may be asymptomatic but still have toxic gentamicin levels and toxicity can develop even in patients with normal gentamicin levels. The best way of avoiding toxicity is to ensure that treatment duration is no more than 3 days unless this is necessary on clinical grounds.
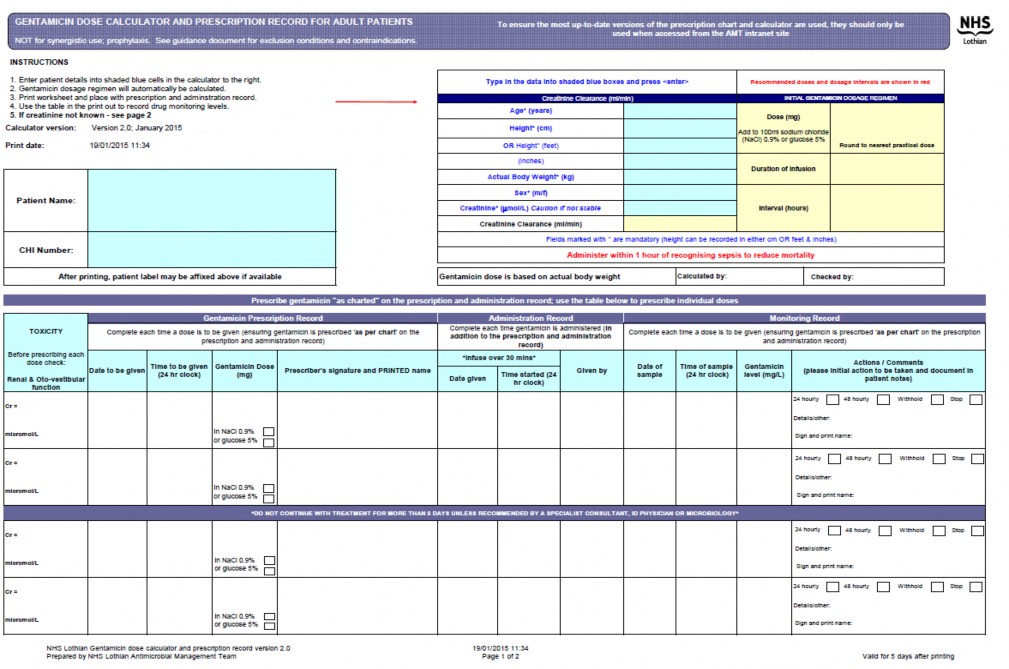
To improve gentamicin prescribing, ensure consistency and reduce risk; print out the calculator worksheet and use to prescribe doses, and record administration and monitoring of gentamicin. Use in conjunction with the inpatient prescribing chart, and medical/nursing documentation.
Start within 1 hour of recognising sepsis; reduces the risk of mortality
Print out a blank copy of the calculator worksheet. See sample at end of this document
Use actual body weight to calculate an initial dose of
o 5mg/kg (max 400mg) OR
o 2.5mg/kg (max 180mg) on advice of senior staff if chronic kidney disease stage 5
Obtain a serum creatinine level and then use calculator to determine dosing regimen
Always use the online calculator, unless the hospital intranet is unavailable.
Print out the calculator worksheet every time. See sample at end of this document.
Patient age, weight, height and serum creatinine are required for dosage calculations.
Obtain a second check for all data entry/calculations before giving the first dose.
Administer calculated dose in 100ml sodium chloride 0.9% as a 30 minute intravenous infusion; ensure time infusion started noted on the calculator sheet.
If the online calculator is not available:
1.1 Use the table below to determine the maximum body weight
Height feet | Height cm | Male MaxBW Kg | Female MaxBW Kg | Height feet | Height cm | Male MaxBW Kg | Female MaxBW Kg | |
4' 8" | 142 | 49 | 43 | 5' 9" | 175 | 85 | 79 | |
4' 9" | 145 | 52 | 47 | 5' 10" | 178 | 88 | 82 | |
4' 10" | 147 | 54 | 49 | 5' 11" | 180 | 90 | 85 | |
4' 11" | 150 | 58 | 52 | 6' 0" | 183 | 94 | 88 | |
5' 0" | 152 | 60 | 55 | 6' 1" | 185 | 96 | 90 | |
5' 1" | 155 | 62 | 58 | 6' 2" | 188 | 98 | 94 | |
5' 2" | 158 | 66 | 60 | 6' 3" | 191 | 101 | - | |
5' 3" | 160 | 68 | 62 | 6' 4" | 193 | 104 | - | |
5' 4" | 163 | 71 | 66 | 6' 5" | 195 | 107 | - | |
5' 5" | 165 | 74 | 68 | 6' 6" | 198 | 109 | - | |
5' 6" | 168 | 77 | 71 | 6' 7" | 201 | 113 | - | |
5' 7" | 170 | 79 | 74 | 6' 8" | 203 | 115 | - | |
5' 8" | 173 | 82 | 77 |
1.2 Is the patient’s actual body weight less than the maximum body weight in the chart above?
2. 1 Is serum creatinine <60micromol/L?
2.2 Calculate creatinine clearance as below:
CrCl (ml/min) = (140 – Age (yrs)) x weight* (Kg) x 1.23 (male) or 1.04 (female) Serum creatinine** (micromol/L)
* actual weight or maximum body weight, whichever is the lower (from section 1.2 above)
** use 60micromol/L if serum creatinine < 60micromol/L
Dose and dose interval are based on calculated creatinine clearance and actual body weight.
To prevent overdosing severely obese patients, doses are capped at a maximum dose.
Inform ward pharmacist of any patient getting more than a single dose of gentamicin.
Obtain a second professional check for all calculations before giving the first dose
Actual Body Weight (Kg) | |||||
Creat Cl (ml/min) | 40 - 49 kg | 50 - 59 kg | 60 - 69 kg | 70 - 80 kg | > 80 kg |
< 21 | 2.5 mg/kg (max 180 mg) then take a sample after 24 hours Seek advice before giving a second dose. | ||||
21 – 30 (48 hour dosing) | 180 mg 48 hourly | 200 mg 48 hourly | 240 mg 48 hourly | 240 mg 48 hourly | 260 mg 48 hourly |
31 – 40 (48 hour dosing) | 200 mg 48 hourly | 240 mg 48 hourly | 280 mg 48 hourly | 300 mg 48 hourly | 320 mg 48 hourly |
41 – 50 (48 hour dosing) | 240 mg 48 hourly | 280 mg 48 hourly | 320 mg 48 hourly | 360 mg 48 hourly | 400 mg 48 hourly |
51 – 60 (24 hour dosing) | 200 mg 24 hourly | 240 mg 24 hourly | 280 mg 24 hourly | 300 mg 24 hourly | 320 mg 24 hourly |
> 60 (24 hour dosing) | 240 mg 24 hourly | 280 mg 24 hourly | 320 mg 24 hourly | 360 mg 24 hourly | 400 mg 24 hourly |
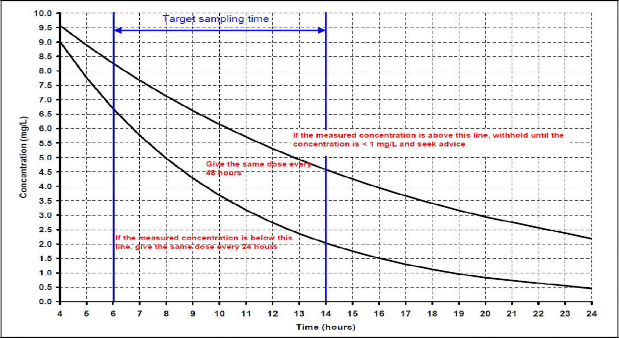
If the patient weighs less than 40 kg and creatinine clearance is ≥ 21 ml/min, give a single dose of 5 mg/kg then take a sample 6 – 14 hours after the dose and plot on the graph.
Add the recommended dose to 100ml sodium chloride 0.9% or glucose 5% and infuse over 30minutes.
Concentrations are meaningless unless dose and sample time are recorded accurately
Print out the calculator worksheet to use as a prescription and monitoring chart, and record:
o Start time of the last dose
o Time blood sample taken
Take a sample 6 – 14 hours after the start of the first infusion and every infusion thereafter
Record the serum concentration on the calculator worksheet
Plot the concentration measurement on the graph; this will indicate one of 3 options:
1) continue the present dosage regimen
2) adjust the dosage interval
3) withhold and resample 24 hours after previous sample
If the result is exactly on the line between two options, choose the option above the line and seek advice from pharmacy.
Seek advice from Pharmacy or Microbiology if you are unsure how to interpret the result or if the concentration is unexpectedly high or low.
If the results are unexpected, take two samples within one dosage interval so that the patient’s actual creatinine clearance and volume of distribution can be calculated.
If a blood sample is omitted, lost or taken at the wrong time take a sample at 20-24 hours after the dose and wait for the result
o If the result is <1mg/L continue existing dosage regimen
o If the result is >1mg/L recheck in 12-24 hours
Monitor creatinine daily

Print out the calculator worksheet to use as a prescription and monitoring chart, and record:
o Start time of the last dose
o Time blood sample taken
Take a blood sample 24 hours after the start of the first gentamicin infusion
Record the serum concentration on the calculator worksheet
Seek advice from Pharmacy or Microbiology if you are unsure how to interpret the result
Monitor creatinine daily
If therapy is to continue, take additional blood samples every 24 hours and give a further dose only when the measured concentration is <1mg/L
Perform pre-prescribing checks to assess the risk of renal toxicity and ototoxicity (see Step 3)
Prescribe the next dose as appropriate
Document the action taken in the medical notes and on the calculator chart
Doses up to 600mg may be required in some patients
Were dose and sample times recorded accurately?
Was the correct dose administered?
Was the sample taken from the line used to administer the drug?
Was the sample taken during drug administration?
Has renal function declined or improved?
Does the patient have oedema or ascites?
If in doubt take another sample before re-prescribing and/or contact pharmacy for advice
Review antimicrobial therapy daily; the risk of toxicity rises when therapy continues beyond 72 hours
o Seek advice from a Microbiologist, ID Consultant or Specialist Consultant if gentamicin is required empirically for >72 hours
o Stop after a maximum of 5 – 7 days unless there is a clear clinical and microbiological need for prolonged therapy.
Consider changing to an oral alternative; refer to the IV to oral switch policy
Monitor renal function by measuring serum creatinine daily
Seek advice if renal function is unstable (e.g. a change in creatinine of >15-20%)
An increase in creatinine or decrease in urine output/oliguria could suggest gentamicin toxicity.
Review therapy with a Microbiologist, ID Consultant or Specialist Consultant with regard to decreasing the dose; and consider an alternative agent
A decrease in creatinine may indicate an improvement in renal function, and the dosage may need to be recalculated
Gentamicin associated ototoxicity is independent of serum drug concentration. It is suggested by any of the following: new tinnitus, dizziness, poor balance, hearing loss or oscillating vision
Toxicity is associated with prolonged aminoglycoside use (usually >10days but may be
>72hours) and is secondary to drug accumulation within the inner ear
Stop treatment if ototoxicity suspected and refer to a Microbiologist, ID Consultant or Specialist Consultant for advice on future therapy
If gentamicin continues beyond 7 days consider referring to audiology for assessment
Thomson AH, Duncan N, Silverstein B, Alcock S, Jodrell D. Development of guidelines for gentamicin dosing. J Antimicrob Chemother 1996;38: 885-893.
Thomson AH, Gourlay Y. Proposal for changes to gentamicin dosage guidelines 2008 (unpublished).
Freeman CD, Nicolau PD, Belliveau PP, Nightingale CH. Once-daily dosing of aminoglycosides: review and recommendations for clinical practice. Journal of Antimicrobial Chemotherapy 1997;39:677–86.
Drusano GL, Ambrose PG, Bhavnani SM et al. Back to the future; using aminoglycosides again and how to dose them optimally. Clinical Infectious Diseases 2007;45:753-60